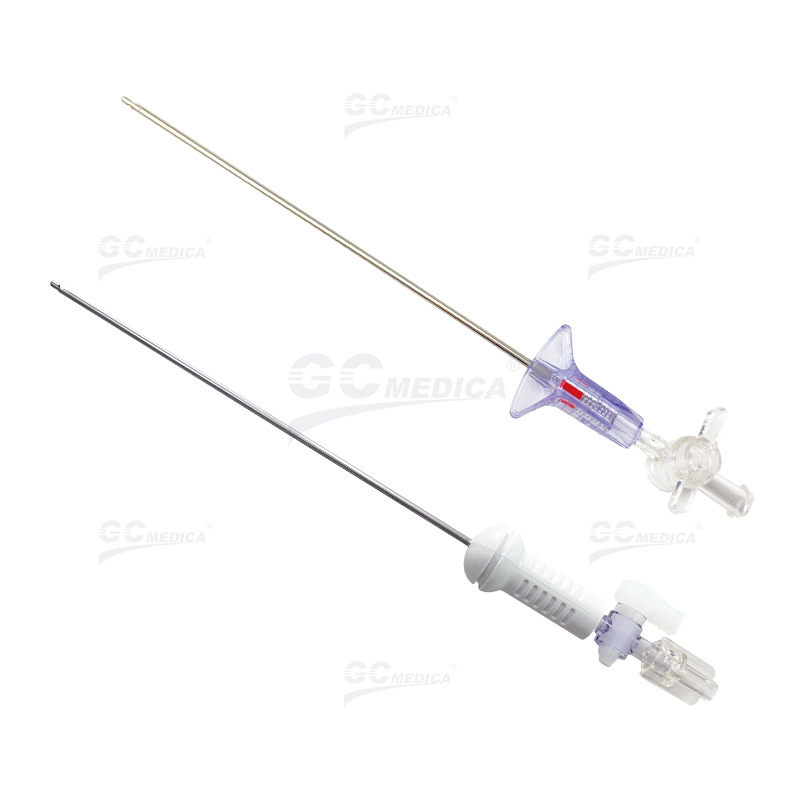
The Veress Needle, a critical instrument in laparoscopic surgeries for creating pneumoperitoneum, requires meticulous procurement strategies. Whether seeking OEM customization or bulk purchasing, understanding the streamlined processes ensures quality and cost-efficiency. This guide outlines key steps for medical suppliers and institutions.
Veress Needle Online Promotion (Click for details >)
OEM Customization Process
1. Requirement Specification & Sampling
Collaborate with manufacturers to define technical specifications (e.g., needle length, diameter) based on surgical needs. For instance, OEM workflows involve:
Design Confirmation: Submit CAD models or technical drawings.
Prototype Testing: Validate functionality (e.g., spring-loaded blunt tip safety).
2. Regulatory Compliance & Production
Ensure adherence to medical device standards (e.g., CFDA, FDA). Manufacturers must provide documentation, including material certifications and sterilization validation. For example, Veress Needles from Kantore Drugstore comply with ISO 13485.
3. Quality Assurance
Post-production inspections (e.g., leak testing, tip sharpness) are mandatory. Suppliers should offer batch-specific test reports.
Bulk Procurement Workflow
1. Supplier Vetting
Identify certified vendors via platforms like B2B portals or industry databases. Prioritize those with proven OEM experience and positive client feedback.
2. RFQ & Price Negotiation
Submit detailed Requests for Quotation (RFQs) covering:
Quantity (e.g., 100–500 units).
Delivery timelines (critical for surgical inventory planning).
Payment terms (e.g., 30% advance, 70% on delivery).
Leverage competitive bidding tools for cost optimization.
3. Order Management
Use ERP systems (e.g., SAP) for PO generation and tracking. For bulk orders exceeding 200 units, negotiate logistics (e.g., FOB or CIF terms).
Key Considerations
Certifications: Verify regulatory approvals (e.g., "Guo Xie Bei" in China).
Supplier Audits: Assess production facilities for GMP compliance.
Post-Sale Support: Ensure warranties and responsive after-sales service.
Conclusion
Efficient procurement of Veress Needles hinges on clear OEM collaboration frameworks and systematic bulk purchasing. By aligning with certified suppliers and leveraging digital tools, healthcare providers can secure high-quality instruments while minimizing operational risks.
Related Products


 Français
Français Español
Español Products
Products

 About Us
About Us